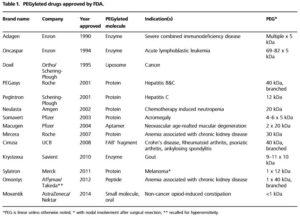
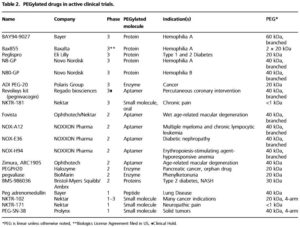
As the first successful technology to improve pharmacokinetic (PK) profiles of therapeutic agents, PEGylation has been widely used to stabilize bioactive molecules (proteins, peptides, enzymes, antibody fragments, oligonucleotides, small synthetic drugs, ets.). As indicated by Prof. Lee in his new manuscript, over 10 PEGylated drugs have been approved by the FDA to date with over 20 drugs in clinical trials and it was estimated that PEGylated drugs would be a multi-billion dollar market.
Since its discovery, PEG chemistry experienced enormous enhancement. First-generation PEGylation involved random PEG conjugation along the biomolecule that showed varying physicochemical and pharmaceutical properties. Second-generation PEGylation took the benefits from an array of PEG derivatives – activated PEG molecules, higher molecular weight PEG polymers and branched PEG structures, which enable to make site-specific PEGylation and further improve the PK profiles. Now third-generation technologies aim to preserve the drug’s bioactivity.
Third-generation PEGylation looks to minimize the current trade-off between potency and circulation half-life for longer-lived drugs. One technique is to utilize customized linker to make releasable PEG conjugates as a predrug approach or create customizable PEGylation sited on proteins. Another approach is to use unique PEG with customized sizes and shapes to affect therapeutic potency of the parent molecule.
Advanced Biochemicals (ABC) offer various linear and branched PEG products. We also provide customer PEG synthesis and customer PEGylation service with different molecular weight and structure .
Original Paper: http://www.tandfonline.com/doi/full/10.1517/14728214.2015.1113254